Georgiou, G., Espeland, L.O., Bukya, H., Vladyslav Yadrykhins’ky, V., Haug, B.E.,Mainkar P.S., Brenk, R.
Towards new antibiotics: P. aeruginosa FabF ligands discovered by crystallographic fragment screening followed by hit expansion.
European Journal of Medicinal Chemistry, 2025, 291, 117563.

Khorsand, F., Haug, B.E., Kursula, I., Reuter, N., Brenk, R.
Expression and purification of human neutrophil proteinase 3 from insect cells and characterization of ligand binding.
PLoS ONE, 2024, 19(6): e0294827.
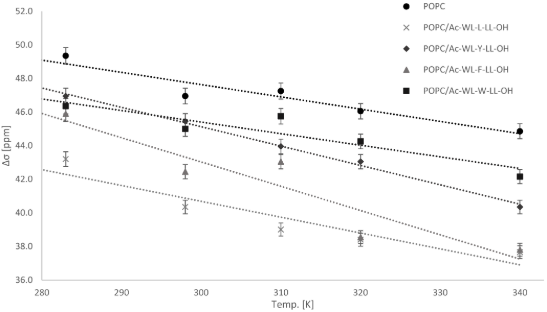
Alsaker, N.E., Halskau, Ø., Haug, B.E., Reuter, N., Nerdal, W.
Phospholipid membrane interactions of model Ac-WL-X-LL-OH peptides
investigated by solid-state nuclear magnetic resonance.
Biomembranes, 2024, 14, 105.
Gartan, P., Khorsand, F., Mizar, P., Vahokovski, J.I., Cervantes, L.F., Haug, B.E., Brenk, R., Brooks III, C.R., Reuter, N.
Investigating Polypharmacology through Targeting Known Human Neutrophil Elastase Inhibitors to Proteinase 3.
Journal of Chemical Information and Modeling 2024, 64, 621–626.

Myklebust, L.M., Baumann, M., Støve, S.I., Foyn, H., Arnesen, T.*, Haug, B.E.*
Optimized bisubstrate inhibitors for the actin N-terminal acetyltransferase NAA80.
Frontiers in Chemistry, 2023, 1202501.
de Jalón, EG., Kleinmanns, K., Fosse, V., Davidson, B., Bjørge, L., Haug, B.E.*, McCormack, E.*
Comparison of five near-infrared fluorescent folate conjugates in an ovarian cancer model.
Molecular Imaging and Biology, 2023, 144-155.

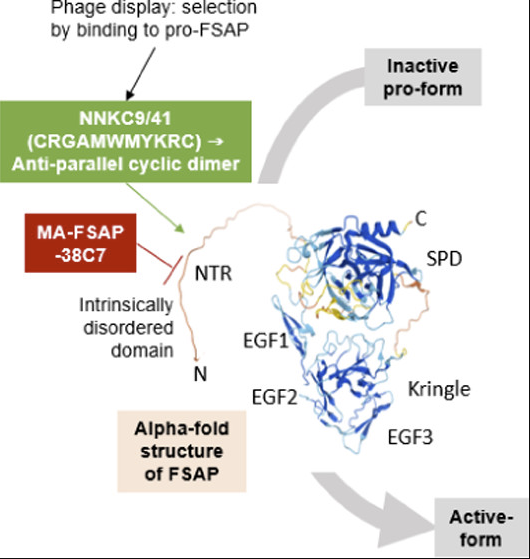
Seidl, S., Nielsen, N.V., Rodriguez, A.A, Escheid, M., Kandanur, S.P.S, Haug, B.E., Stensland, M., Thiede, B., Karacan, M., Preising, N., Wiese, S., Ständker, L., Declerck, P.J., Løset, G.Å., Kanse, S.M.
Identification of a phage display-derived peptide interacting with the N-terminal region of Factor VII activating protease (FSAP) enables characterization of zymogen activation.
ACS Chemical Biology, 2022, 17, 2631-2642.
Espeland, L.O., Georgiou, C., Klein, R., Bhukya, H., Haug, B.E., Underhaug, J., Mainkar, P.S., Brenk, R.
An experimental toolbox for structure-based hit discovery for P. aeruginosa FabF, a promising target for antibiotics.
ChemMedChem, 2021, 16(17), 2715-2726.
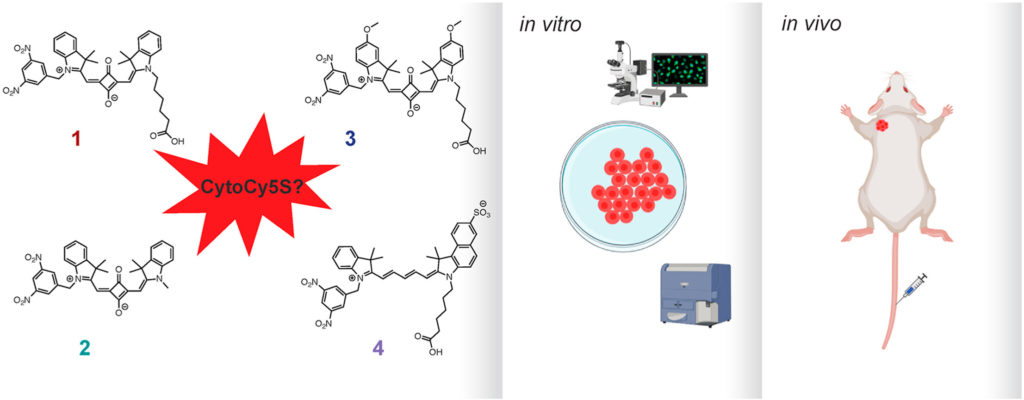
de Jalón, E.G., de Garibay, G.R., Haug, B.E.*, McCormack, E*.
CytoCy5S™, a compound of many structures. in vitro and in vivo evaluation of four near-infrared fluorescent substrates of nitroreductase (NTR).
Dyes and Pigments, 2021, 196, 109553.
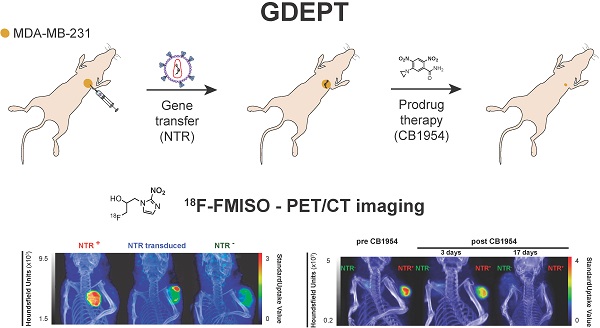
de Garibay, G.R., de Jalón, E.G., Stigen, E., Lund, K.B., Popa, M., Davidson, B., Safont, M.M, Rygh, C.B., Espedal, H., Barrett, T.M., Haug, B.E., McCormack, E.
Repurposing 18F-FMISO as a PET tracer for translational imaging of nitroreductase-based gene directed enzyme prodrug therapy.
Theranostics, 2021, 11(12), 6044-6057.
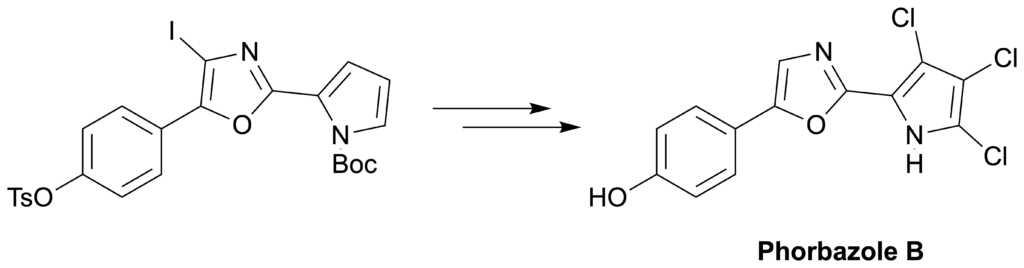
Guttormsen, Y., Fairhurst, M. E., Pandey, S. K, Isaksson, J., Haug, B. E.*, Bayer, A.*
Total synthesis of phorbazole B.
Molecules, 2020, 25 (20), 4848.
Ndukwe, I. E., Lam, Y.-H., Pandey,S. K., Haug, B. E., Bayer, A., Sherer, E. C., Blinov, K. A., Williamson, R. T., Isaksson, J., Reibarkh, M., Liu, Y., Martin, G. E.
Unequivocal Structure Confirmation of a Breitfussin Analog by Anisotropic NMR Measurements.
Chemical Science, 2020, 11, 12081-12088.
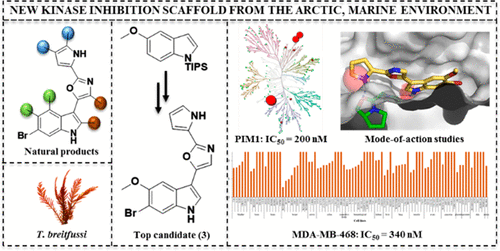
Hansen, K. O.*, Andersen, J. H., Bayer, A., Pandey, S. K., Lorentzen, M., Jorgensen, K. B., Sydnes, M. O., Guttormsen, Y., Baumann, M., Koch, U., Klebl, B., Eickhoff, J., Haug, B. E.*, Isaksson, J., Hansen, E. H.
Kinase Chemodiversity from the Arctic: The Breitfussins.
Journal of Medicinal Chemistry, 2019, 62 (22), 10167-10181.
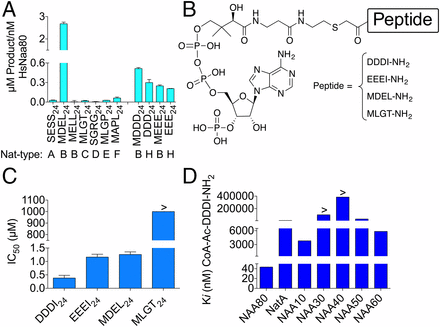
Goris, M., Magin, R. S., Foyn, H., Myklebust, L. M., Drazic, A., Bhambra, P., Varland, S., Støve, S. I., Baumann, M., Haug, B. E., Marmorstein, R., Arnesen, T.
Structure, substrate specificity and selective inhibition of the actin N-terminal acetyltransferase Naa80.
Proceedings of the National Academy of Sciences (USA), 2018, 115 (17), 4405-4410.
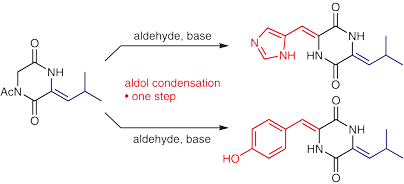
Fairhurst, M. E., Zeeshan, M., Haug, B.E.*, Bayer, A.*
Aldol condensations on a 3-alkylidene-2,5-diketopiperazine – synthesis of two marine natural products.
Synlett, 2018, 29 (10), 1303-1306.
Nestvold, J., Wang, M.-Y., Camilio, K. A., Tjelle, T. E., Lindberg, A., Haug, B. E., Kvalheim, G., Sveinbjørnsson, B., Rekdal, Ø.
Oncolytic peptide LTX-315 induces an immune-mediated abscopal effect in a rat sarcoma model.
OncoImmunology, 2017, 6 (8), e1338236.
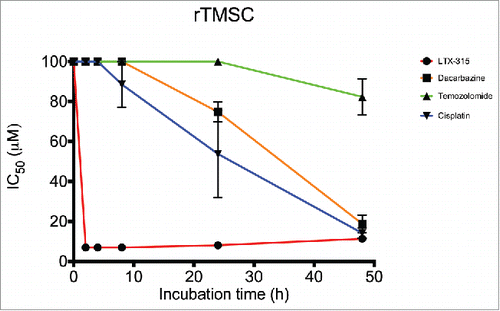
Baumann, M., Nome, L. M., Zachariassen, Z. G., Karlshøj, S., Fossen, T., Rosenkilde, M. M., Våbenø, J.*, Haug, B. E.*
Synthesis of a novel Tripeptidomimetic scaffold and biological evaluation for CXC chemokine receptor 4 (CXCR4) antagonism.
Tetrahedron, 2017, 73 (27), 3866-3877.
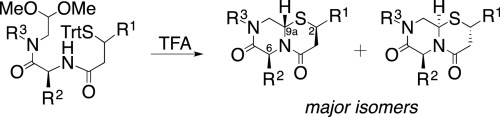
Sveinbjørnsson, B., Camilio, K. A., Haug, B. E., Rekdal, Ø.
LTX-315: A first in class oncolytic peptide that reprograms the tumor microenvironment.
Future Medicinal Chemistry, 2017, 9 (12), 1339-1344.
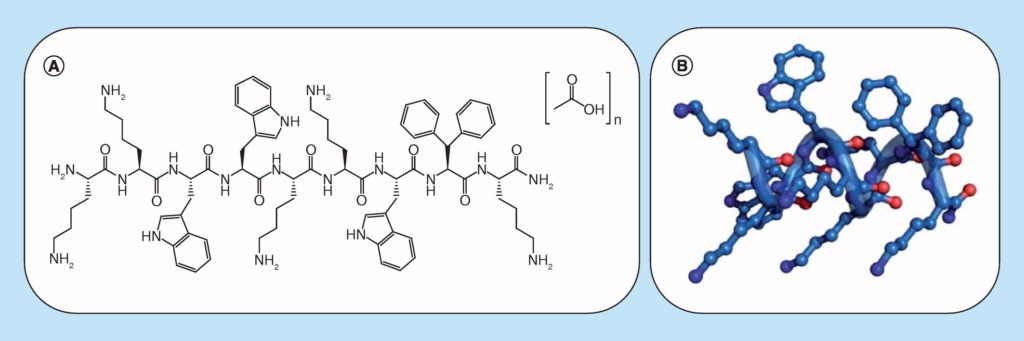
Baumann, M., Hussain, M. M., Henne, N., Garrote, D. M., Karlshøj, S., Fossen, T., Rosenkilde, M. M., Våbenø, J.*, Haug, B. E.*
Influence of chain length on the activity of tripeptidomimetic antagonists for CXC chemokine receptor 4 (CXCR4).
Bioorganic & Medicinal Chemistry, 2017, 25 (2), 646-657.
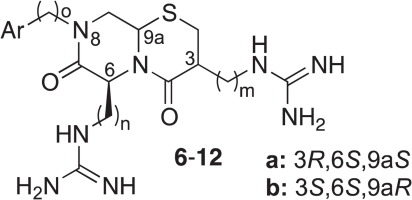
Støve, S. I., Magin, R. S., Foyn, H., Haug, B. E., Marmorstein, R., Arnesen, T.
Crystal structure of the Golgi-associated human N-alpha acetyltransferase 60 (Naa60/NatF) reveals the molecular determinants for substrate-specific acetylation.
Structure, 2016, 24 (7), 1044-1056.
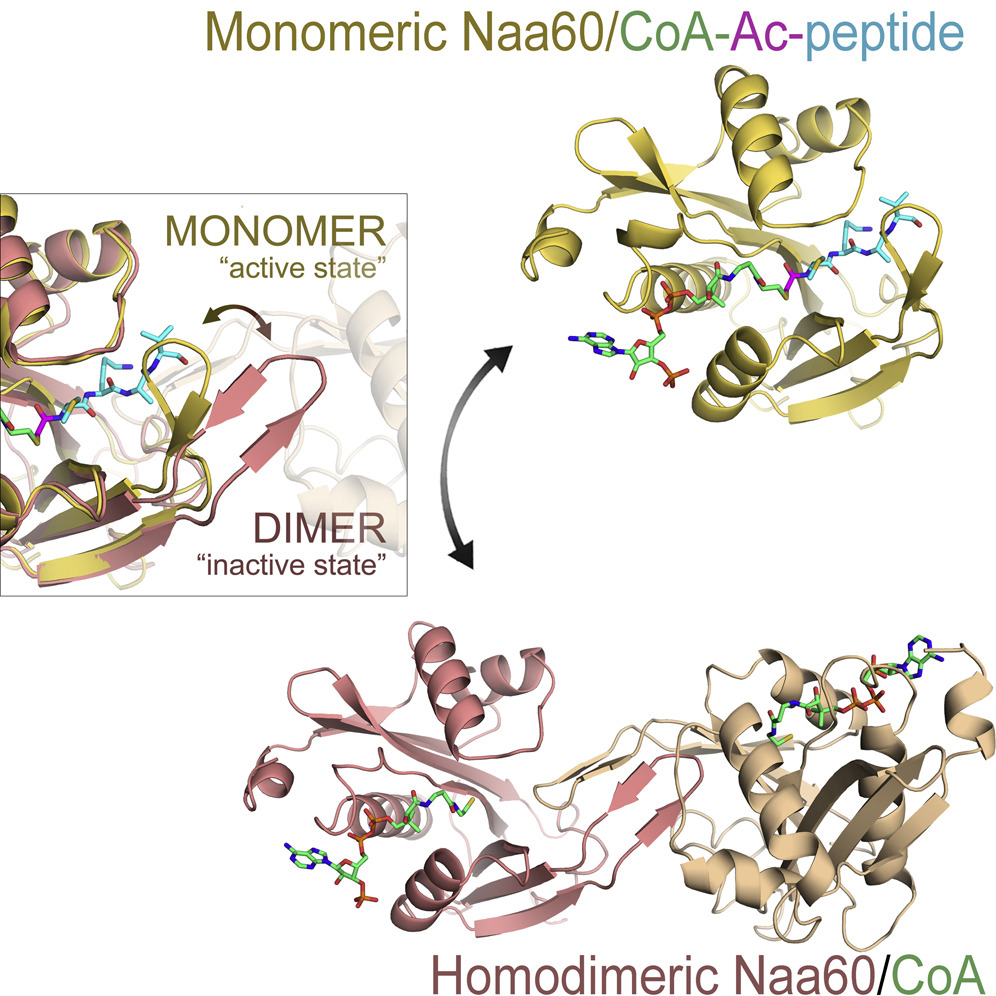
Haug, B. E.*, Camilio, K. A., Eliassen, L. T., Stensen, W., Svendsen, J. S., Berg, K., Mortensen, B., Serin, G., Mirjolet, J.-F., Bichat, F., Rekdal, Ø.*
Discovery of a 9-mer cationic peptide (LTX-315) as a potential first in class oncolytic peptide.
Journal of Medicinal Chemistry, 2016, 59 (7), 2918-2927.
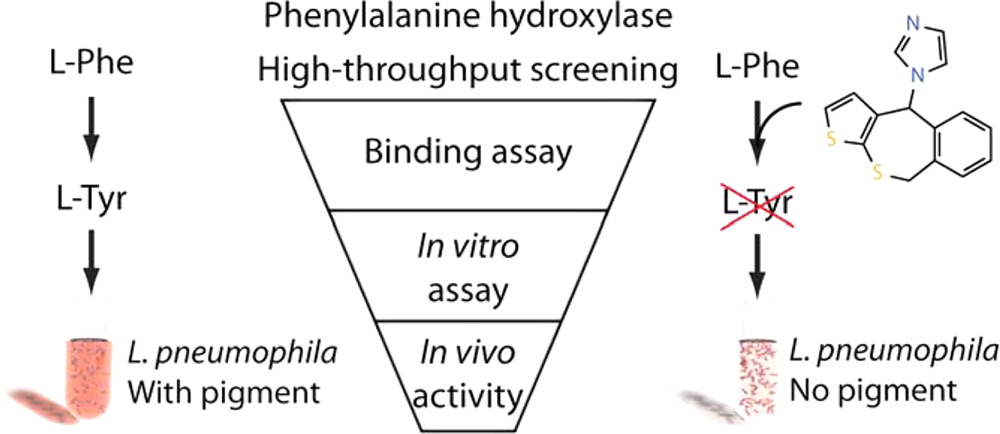
Aubi, O., Flydal, M. I., Zheng, H., Skjærven, L., Rekand, I., Leiros, H.-K. S., Haug, B. E., Cianciotto, N. P., Martinez, A., Underhaug, J.
Discovery of a specific inhibitor of pyomelanin synthesis in Legionella pneumophila.
Journal of Medicinal Chemistry, 2015, 58 (21), 8402-8412.
Zachariassen, Z. G., Karlshøj, S., Haug, B. E., Rosenkilde, M. M., Våbenø, J.
Probing the molecular interactions between CXC chemokine receptor 4 (CXCR4) and an arginine-based tripeptidomimetic antagonist (KRH-1636).
Journal of Medicinal Chemistry, 2015, 58 (20), 8141-8153.
Våbenø, J., Haug, B. E., Rosenkilde, M. M.,
Progress toward rationally designed small-molecule peptide and peptidomimetic CXCR4 antagonists.
Future Medicinal Chemistry, 2015, 7(10), 1261-1283.
Liu, L., Budnjo, A., Jokela, J., Haug, B. E., Fewer, D., Wahlsten, M., Rouhiainen, L., Permi, P., Fossen, T., Sivonen, K.
Pseudoaeruginosins, nonribosomal peptides in Nodularia spumigena.
ACS Chemical Biology, 2015, 10 (3), 725-733.
Pandey, S. K., Guttormsen, Y., Haug, B. E., Hedberg, C., Bayer, A.
A concise total synthesis of breitfussin A and B.
Organic Letters, 2015, 17 (1), 122-125.
Budnjo, A., Narawane, S., Grauffel, C., Schillinger, A.-S., Fossen, T., Reuter, N., Haug, B. E.
Reversible ketomethylene-based inhibitors of human neutrophil proteinase 3.
Journal of Medicinal Chemistry, 2014, 57, 9396-9408.
Zachariassen, Z. G., Thiele, S., Berg, E. A., Rasmussen, P., Fossen, T., Rosenkilde, M. M., Våbenø, J., Haug, B. E.
Design, synthesis, and biological evaluation of scaffold-based tripeptidomimetic antagonists for CXC chemokine receptor 4 (CXCR4).
Bioorganic & Medicinal Chemistry, 2014, 22, 4759-4769.
Narawane, S., Budnjo, A., Grauffel, C., Haug, B. E., Reuter, N.
In silico design, synthesis, and assays of specific substrates for proteinase 3: Influence of fluorogenic and charged groups.
Journal of Medicinal Chemistry, 2014, 57 (3), 1111–1115.
Harmsen, R. A. G., Sivertsen, A., Michetti, D., Brandsdal, B. O., Sydnes, L. K., Haug, B. E. Synthesis and docking of novel piperidine renin inhibitors.
Monatshefte für Chemie, 2013, 144(4), 479-494.
McCormack, E., Silden, E., West, R. M., Pavlin, T., Micklem, D. R., Lorens, J., Haug, B. E., Cooper, M. E., Gjertsen, B. T.
Nitroreductase, a near infrared reporter platform for in vivo time-domain optical imaging of metastatic cancer.
Cancer Research, 2013, 73(4), 1276-1286.
Steinkopf, S., Hanekam, L., Schaathun, M., Budnjo, A., Haug, B. E., Nerdal, W.
Interaction of local anesthetic articaine enantiomers with brain lipids: A Langmuir monolayer study.
European Journal of Pharmaceutical Sciences, 2012, 47(2), 394-401.
Farooq, T., Sydnes, L. K., Törnroos, K. W., Haug, B. E.
Debenzylation of functionalized N-benzyl 4- and 5-substituted 1,2,3-triazoles.
Synthesis, 2012, 44, 2070-2078.
Farooq, T., Haug, B. E., Sydnes, L. K., Törnroos, K. W.
1,3-Dipolar cycloaddition of benzyl azide with two highly functionalized alkynes.
Monatshefte für Chemie, 2012, 143(3), 505-512.
Rekdal, Ø., Haug, B. E., Kalaaji, M., Hunter, H. N., Lindin, I., Israelsson, I., Solstad, T., Yang, N., Brandl, M., Mantzilas, D., Jing, W., Vogel, H.
The relative spatial positions of tryptophan and cationic residues in helical membrane-active peptides determines their cytotoxicity.
Journal of Biological Chemistry, 2012, 287(1), 233-244.
Harmsen, R. A. G., Sydnes, L. K., Törnroos, K. W., Haug, B. E.. Synthesis of trans-4-triazolyl-substituted 3-hydroxypiperidines.
Synthesis, 2011, 5, 749-754.
Skjevik, Å. A., Haug, B. E., Lygre, H., Teigen, K.
Intramolecular hydrogen bonding in articaine can be related to superior bone tissue penetration. A molecular dynamics study.
Biophysical Chemistry, 2011, 154(1), 18-25.
Haug, B. E., Kalaaji, M., Rekdal, Ø., Stensen, W., Svendsen, J.S.
Synthetic antimicrobial peptidomimetics with therapeutic potential.
Journal of Medicinal Chemistry, 2008, 51(14), 4306-4314.
Svenson, J., Brandsdal, B. O., Stensen, W., Haug, B. E., Svendsen, J. S.
Antibacterial peptides with stability towards tryptic degradation.
Biochemistry, 2008, 47(12), 3777-3788.
Haug, B. E.*, Stensen, W., Svendsen, J. S. Application of the Suzuki-Miyaura cross-coupling to increase antimicrobial potency generates promising novel antibacterials.
Bioorg. Med. Chem. Lett. 2007, 17(8), 2361-2364.
Haug, B. E., Strøm, M. B., Svendsen, J. S. The medicinal chemistry of short lactoferricin-based antibacterial peptides.
Curr. Med. Chem. 2007, 14(1), 1-18.
Haug, B. E., Brewer, M., Rich, D. H. Facile degradative lactonization of Gln-Arg and Gln-Phe hydroxyethylene dipeptide derivatives.
J. Peptide. Res., 2005, 65(1): 77-83.
Haug, B. E., Rich, D. H. Synthesis of a Gln-Phe hydroxyethylene dipeptide isostere.
Org. Lett., 2004, 6(25): 4783-4786.
Haug, B. E., Stensen, W., Stiberg, T., Svendsen, J. S. Bulky non-proteinogenic amino acids permit the design of very small and effective cationic antibacterial peptides.
J. Med. Chem. 2004, 47(17): 4159-4162.
Eliassen, L. T., Haug, B. E., Berge, G., Rekdal, Ø. Enhanced antitumour activity of 15-residue bovine lactoferricin derivatives containing bulky aromatic amino acids and lipophilic N-terminal modifications.
J. Peptide Sci, 2003, 9(8): 510-517.
Strøm, M. B., Haug, B. E., Skar, M. L., Stensen, W., Stiberg, T., Svendsen, J. S. The pharmacophore of short cationic antibacterial peptides.
J. Med. Chem. 2003, 46(9): 1567-1570.
Haug, B. E., Andersen, J., Rekdal, Ø., Svendsen, J. S. Synthesis of a 2-arylsulfonylated tryptophan: The antibacterial activity of bovine lactoferricin peptides containing Trp(2-Pmc).
J. Peptide Sci. 2002, 8 (7): 307-313.
Lejon, T., Svendsen, J. S., Haug, B. E. Simple parameterisation of non-natural amino acids for QSAR of antibacterial peptides.
J. Peptide Sci. 2002, 8 (7): 302-306.
Strøm, M. B, Haug, B. E., Rekdal, Ø., Skar, M. L., Stensen, W., Svendsen, J. S. Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity.
Biochem. Cell. Biol. 2002, 80(1): 65-74.
Haug, B. E., Skar, M. L., Svendsen, J. S. Bulky aromatic amino acids increase the antibacterial activity of bovine lactoferricin peptides.
J. Peptide Sci. 2001, 7(8): 425-432.
Haug, B. E., Svendsen, J. S. The role of tryptophan in the antibacterial activity of a 15-residue bovine lactoferricin peptide.
J. Peptide Sci. 2001, 7(4): 190-196.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.